September 5, 2024
Novel Anti-obesity Drugs And Plasma Lipids Web Page 3
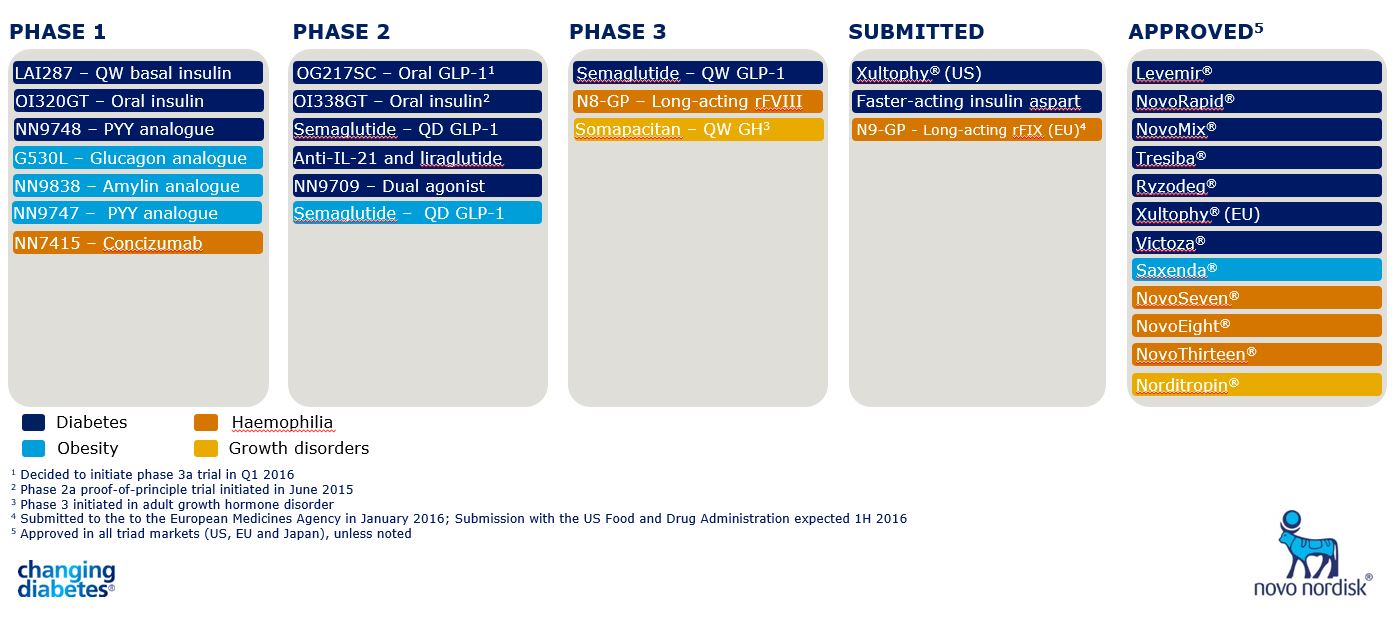
Long-lasting Efficacy And Safety Of Anti-obesity Therapy: Where Do We Stand? Current Excessive Weight Reports The stomach-derived peptide hormone ghrelin reaches the hypothalamus using the typical renown and boosts homeostatic food consumption with activation of NPY/AgRP neurons245, while stimulating hedonic consuming with activation of dopaminergic neurons in the forward tegmental area302. To activate its receptor, ghrelin calls for N-octanoylation (acylation) at its serine 3 residue, and as dietary lipids are used for ghrelin acylation, this recommends that ghrelin may also serve as a nutrient sensing unit that educates the mind concerning incoming nutrients245. This section on future anti-obesity medications focuses on tesofensine, since itis the only CNS acting anti-obesity medicine that has actually gotten to a sophisticated stage ofdevelopment. All other CNS acting drugs remain in early in scientific growth andother than the restricted details on semaglutide and setmelanotide have actually nopublished trials for obesity therapy [112] Aminorex was authorized for non-prescription sale as a treatment ofobesity in Austria, Switzerland and West Germany in 1965, yet was never approvedin the United States [9]Food Consumption And Body Weight
What is the new scientist obesity drug?
New study is revealing the unexpected mind and psychological wellness benefits of semaglutide medicines such as Ozempic and Wegovy, and various other related diabetes and weight-loss medicines that imitate a digestive tract hormonal agent launched after consuming.
1 Glucagon-like Peptide 1 + Glucagon Receptor Agonists
The cetilistat team lost 3.85-- 4.32 kg, comparable to the 3.78 kg weight management of the orlistat group [74] Nevertheless, there are no researches on the lasting effects of cetilistat on weight management and security. Because 1959, phentermine has actually been utilized for short-term weight control, which is permitted only for much less than 12 weeks due to the lack of lasting safety data [30]- The objective of anti-obesity treatment is finding compounds that are effective and have minimal negative effects.
- However, whereas weight loss results typically convert from rats to human beings, optimum effectiveness is historically 2 to four times lower in people about rats (Fig. 3).
- Fat burning depended on 10.6% in people, which was roughly two times the weight reduction generated by drugs currently accepted by the United States FDA for treating obesity.
- This kind of growth most often affects the physiological feature of the hypothalamus, a component of the mind that controls cravings and metabolic process, therefore bring about quick, intractable weight gain, a condition called hypothalamic obesity [50]
- Given that sleep is thought about to be a period of power preservation, hypersomnia in clients with hypothalamic damages can lead to a reduction in energy expense (58 ).
3 Methionine Aminopeptidase Inhibitors (metap
Receptor villains were included succeeding experiments thatmeasured acute hypophagia over the initial 12 hours of tesofensine therapy. Anα1-adrenoreceptor villain eliminated most of the hypophagia and a D1dopamine receptor antagonist revealed partial View website restraint. Antagonists of theα2-adrenoreceptor, dopamine D2, dopamine D3, and serotonin 2A/C receptorsdid not lower tesofensine activity [118] A stage II dose-ranging research of liraglutide was performed in obese subjectsto take a look at the results on food consumption and body weight. High blood pressure wasreduced in all liraglutide groups from baseline and the frequency ofpre-diabetes in the 3mg group was decreased by 96%. One of the most constant adverseevents were queasiness and throwing up which were mainly short-term and seldom led todiscontinuation [89] Furthermore, boosting rates of youth excessive weight are likely to intensify the trend towards raising obesity in the adult years. The procedure of the very first Phase III trial was approved by the US Food and Drug Administration in the very first fifty percent of 2010. Tesofensine has a long half-life of concerning 9 days (220 h) [4] "and is mostly metabolized by cytochrome P4503A4 (CYP3A4) to its desalkyl metabolite M1" NS2360. [10] [11] NS2360 is the only metabolite detectable in human plasma. It has a longer half-life than tesofensine, i.e. approximately 16 days (374 h) in human beings, and has an exposure of 31-- 34% of the moms and dad substance at stable state. In vivo information indicate that NS2360 is responsible for approximately 6% of the activity of tesofensine. 
Social Links