
September 5, 2024
Pharmaceuticals Totally Free Full-text Present Treatments In Professional Tests Of Parkinsons Disease: A 2021 Upgrade

Obesity: The 21st Century Epidemic
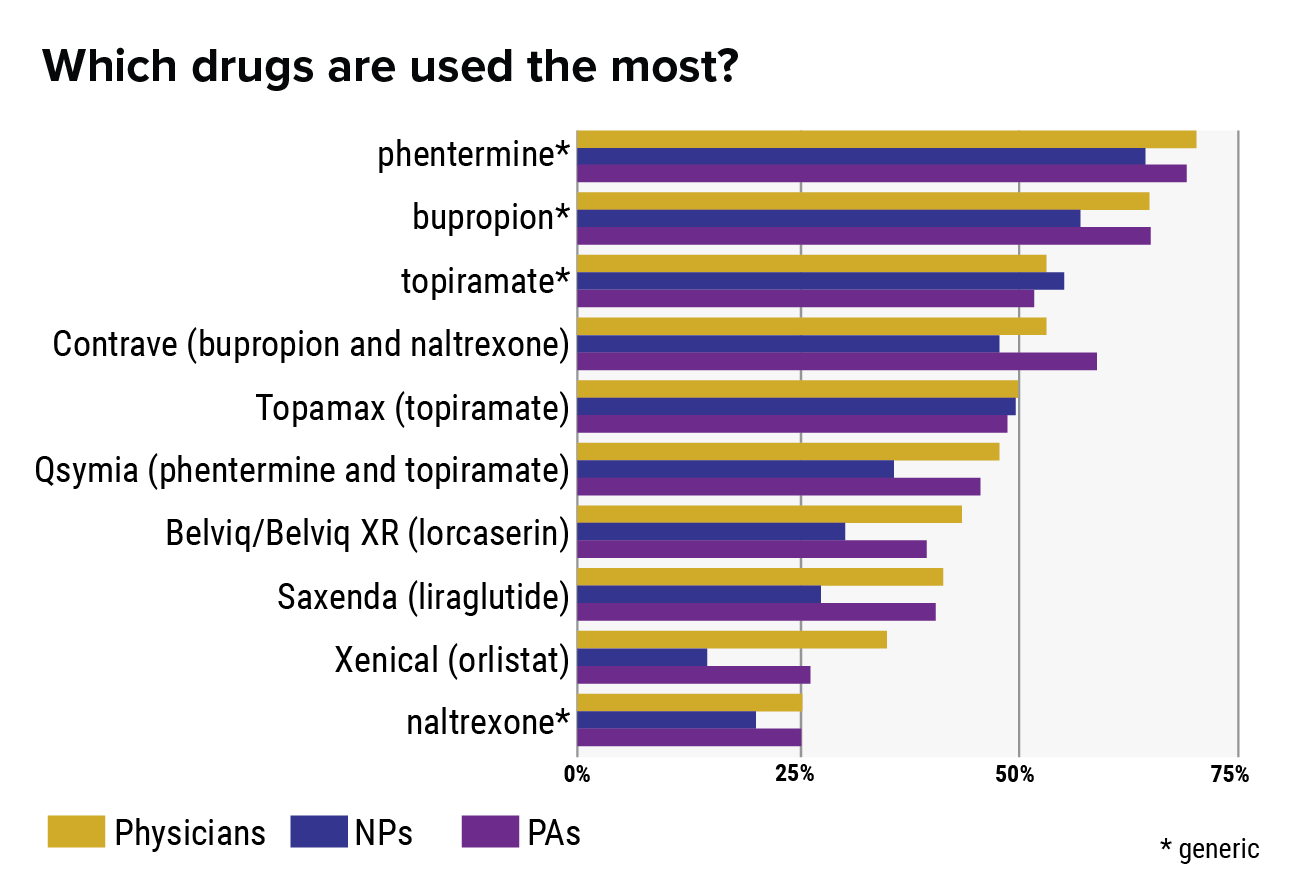
Which drug works best for weight reduction?
Some preferred weight-loss tablets are Contrave (naltrexone/ bupropion), Qsymia (phentermine/ topiramate ER), and phentermine (Adipex-P). Some clinical researches suggest that Qsymia is the most effective weight loss pill.
Tesofensine Peptide: Dopamine-serotonin, Noradrenaline Reuptake Inhibitor
The weight problems dilemma has reached the stage that strong consideration ought to be offered to adequate application of this efficient and economical course of medicine. Professional tests assessing the efficiency of Tesofensine for weight-loss have actually produced encouraging outcomes. In one research study entailing obese people, Tesofensine therapy resulted in a mean fat burning of roughly 10% of first body weight after 24 weeks of therapy. However, the overall fat in the Chow-Tesofensine group did not vary considerably from that of the Chow-Saline team. These results show that tesofensine reduced complete visceral fat, primarily mesenteric fat deposits, in overweight rats. This would additionally limit the capacity for abuse and unfavorable long-lasting cardiovascular results. Amylin Drug Inc's., Pramlintin, (Symlin), based upon the gut hormonal agent amylin is authorized for the treatment of diabetes mellitus.- The primary endpoint was safety; secondary endpoints included steps of body weight, appetite scores, lifestyle, and metabolic profile.
- Provided the power of the technique, multi-agonism treatment has been repetitively used in preclinical therapy of obesity, generally however not exclusively in mix with some kind of GLP1 agonism.
- Obesity-related expenses to the US medical care system have actually increased in the last years to as much as $147 billion, according to a recent research commissioned by the Centers for Illness Control and Prevention (CDC).
- In the Tesomet group, one client created anxiousness related to Tesomet and the other had recurrence of craniopharyngioma with succeeding post-procedural complications to surgery unassociated to Tesomet.
- Last but not least, the synchronised contrast of peptides matched in structure and pharmacokinetics, however otherwise without a solitary biological task, makes up an expensive financial investment when the length of research study is gauged in months.
- In one research study including overweight people, Tesofensine treatment led to a mean weight loss of roughly 10% of initial body weight after 24 weeks of therapy.
Tesofensine Reduced Feeding Habits Induced By Optogenetic Activation Of Lh Gabaergic Nerve Cells In Lean Vgat-chr2 Mice
Preliminary research study suggests increased activity in central places of importance to weight control123. Nonetheless, this is just a start and a much deeper molecular understanding may Check over here lead to also additional enhancements in GLP1R agonists, or various other representatives that could act by an independent mechanism at comparable anatomical websites. Trial timeline of a randomized regulated test of Tesomet for weight management in with hypothalamic excessive weight. Follow-up to address negative occasions after end of treatment was carried out 45 ± 3 days after the last dose of investigational product. Several various other therapy choices versus obesity are being researched in professional tests, including cannabinoid type 1 receptor blockers, amylin mimetics, peptide YY, neuropeptide Y inhibitors, fibroblast development variable 21 analogs, and vaccinations [36] It is feasible that the management of a specific combination of these drugs will certainly have valuable impacts on the complicated physical factors that contribute to the upkeep body weight. A caution to this last finding is that the reduction of YFAS scores within 24 h may be faster than pexacerfont's predicted time course of CNS action. Generally, the results give reasoning for well-powered tests of CRF1 receptor antagonists to decrease uncontrollable consuming (Epstein et al., 2016; Spierling and Zorrilla, 2017). Given that tesofensine is a three-way reuptake inhibitor that manages the level of DA, 5-HT, and NE throughout the whole brain, its results are expected to be dispersed and brain-wide, certainly not limited to LH or GABAergic nerve cells. Further studies using high-density recordings of neuropixels require to unveil exactly how distributed tesofensine's impacts are throughout the mind. Hereof, the equilibrium of neurotransmitters in the brain, particularly norepinephrine (NE), dopamine (DA), and serotonin (5-HT), is a major determinant of the overall weight loss homes of a lot of appetite suppressants [14, 25, 64] A caution of our research study is that we did not determine the launch of these natural chemicals.Social Links