September 5, 2024
Tesofensine, An Unique Antiobesity Medicine, Silences Gabaergic Hypothalamic Nerve Cells Pmc
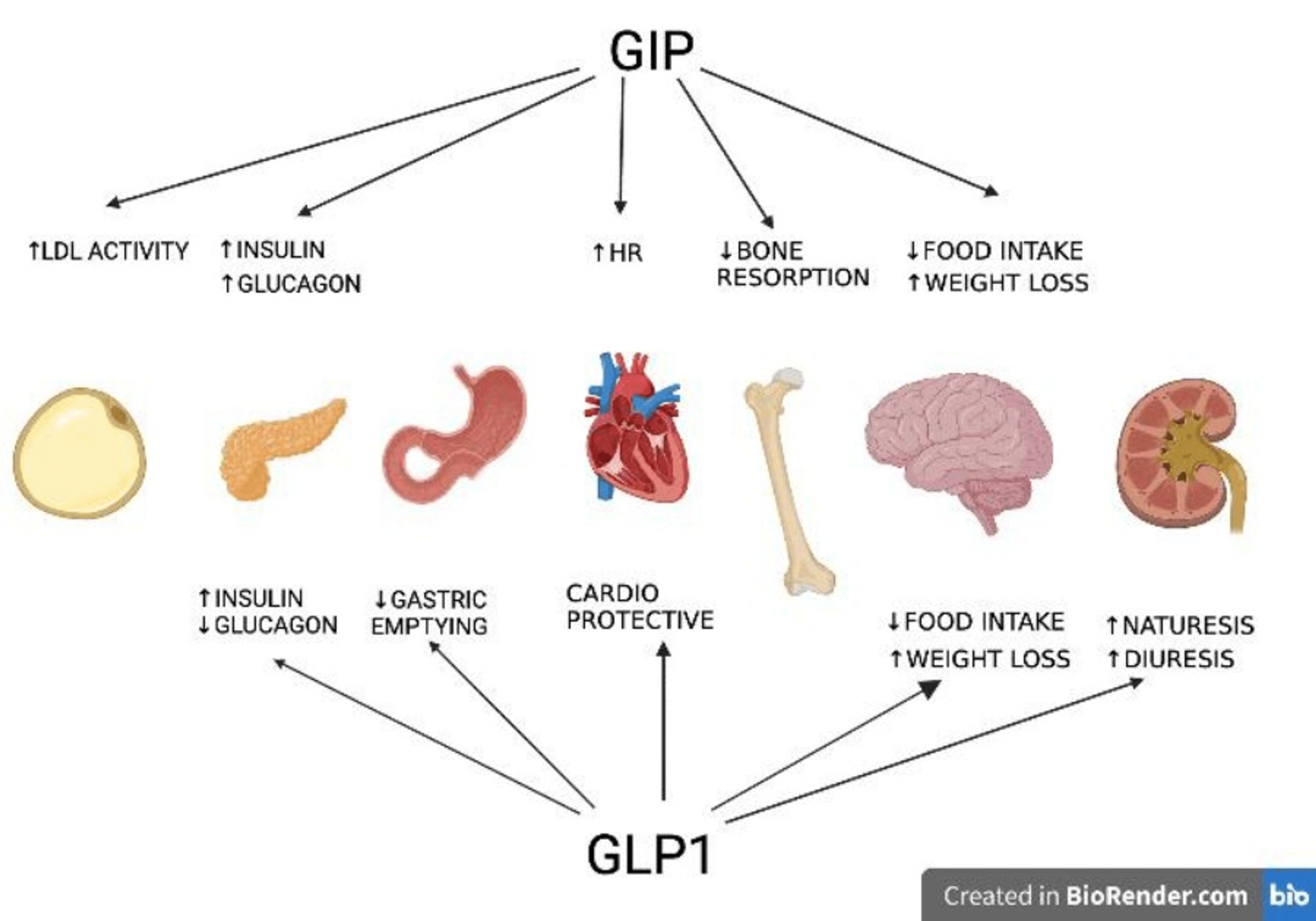
Pharmacotherapy For Obesity Web Page 5 Main Outcome Actions Coprimary end factors were the changes from standard in Unified Parkinson Disease Score Scale (UPDRS) subscale II (activities of day-to-day living) plus subscale III (motor function) complete score and in portion of waking hours spent in "off" time noted in self-scoring journals. Second end points were safety, pharmacokinetics, responder evaluation (≥ 20% decrease in UPDRS score and in off time), and modifications in percentage of waking hours spent in "on" time with and without bothersome dyskinesia. Plasma concentrations of tesofensine (NS 2330) are revealed as the mean focus for every treatment team at the time points indicated. The recent advancements in our understanding of the centrally mediated pathways pertinent to power and appetite law have actually resulted in a targeted pharmacological method in an effort to bypass damaged hypothalamic paths.5 Bupropion And Naltrexone (contrave)
- We will after that define the anti-obesity medications readily available today thatact on the mind, and wrap up with a review of the potential of brand-new centrallyacting medications in professional advancement.
- In phase III scientific trials, Contrave demonstrated that people on a diet regimen and exercise program accomplished greater weight reduction over 56 weeks with bupropion/naltrexone (6.1 kg) than with placebo (1.4 kg) (Orexigen, 2010).
- Such a tri-agonist has actually shown excellent guarantee in pet screening and progressed to professional studies210,211.
- Topiramate development as a medication for the therapy ofobesity was discontinued as a result of the unfavorable events.
Medicine Release Profile Of An Unique Exenatide Lasting Drug Delivery System (okv- Carried Out To Pet Cats
Damaging results of zonisamide, such as anxiety and sedation, might relapse by its combination with bupropion (Ioannides-Demos et al., 2011). A 24-wk Phase II scientific test of the continual launch formulation of bupropion (360 mg)- zonisamide (360 mg) combination generated higher weight loss (9.2%) than bupropion (6.6%) or zonisamide (3.6%) alone or compared to placebo (0.4%) (Ioannides-Demos et al., 2011). Stage III clinical tests with the dealt with dosage mix are underway (George et al., 2014). Contrave is a combination of bupropion and naltrexone in a sustained-release formula and is presently in the procedure of resubmission after the FDA decreased to accept the drug in 2011, citing security worries at the time.What class of medicine is tesofensine?
Tesofensine is a Serotonin-norepinephrine-dopamine-reuptake-inhibitor (SNDRI). SNDRIs are a class of psychedelic antidepressants. They act upon neurotransmitters in the mind, particularly, serotonin, norepinephrine and dopamine.

Social Links