
August 27, 2024
Bpc-157
Body Protective Compound-157 Boosts Alkali-burn Injury Recovery In Viv Dddt Direct partnerships were observed in between AUC0-- t and BPC157 dosages, along with between Cmax and BPC157 dosages (Figures 2D, E). The outright bioavailability observed after IM administration of each dosage in canines was 45.27%, 47.64%, and 50.56%, respectively. After repeated IM administration of BPC157 at 30 μg/ kg for seven consecutive days, the plasma focus versus time curve resembled that observed after a single IM injection of 30 μg/ kg (Figure 2C). Nonetheless, the pharmacokinetic specifications after repeated IM administration altered somewhat compared to those observed after a solitary IM shot, with a little decline in Cmax and t1/2 and a rise in Tmax.Dispute Around Fda's Bpc 157 Restriction
- While these researches recommend that BPC-157 might have anti-tumor residential properties, much more considerable study, including scientific tests, is necessary to completely recognize its potential and devices of activity in cancer treatment.
- Widely talked about due to its appeal, this development has actually opened up a series of viewpoints and discussions.
- Teams 2, 3, and 4 were provided 20, 100, and 500 μg/ kg BPC157 saline solutions through solitary IM injections, respectively.
- BPC 157 acts as a membrane stabilizer and complimentary radical scavenger and decreases leaking digestive tract disorder, as received stomach tract cytoprotective studies (Park et al., 2020).
- Continually, with intensifying (obtained with L-NAME administration) and amelioration (with L-arginine), either L-arginine-amelioration dominates (i.e., esophageal and gastric lesions undermined) or they counteract each various other (L-NAME + L-arginine) with an impact that was further turned around towards a significant helpful impact by the enhancement of BPC 157 (L-NAME + L-arginine + BPC 157).
Mapping The Exploration Of Bpc-157 In Scientific Studies
This step makes certain specific health elements and possible medication interactions get mindful consideration. Resolving the efficiency of this powerful peptide entails an analysis of the outcomes garnered from various approaches of delivery, varying from injections to dental applications, each research study contributing to a more complete understanding of BPC-157's duty in physical reconstruction. A deeper query into BPC-157 introduces its function in the orchestration of cellular dynamics, which ignites healing. After solitary IM administrations of dosages 20, 100, or 500 μg/ kg, the peak time (Tmax) of each dose was 3 minutes. The maximum focus (Cmax) of each dose were 12.3, 48.9, and 141 ng/ml, specifically, and the AUC0-- t values were 75.1, 289, and 1930 ng min/ml, specifically. Linear partnerships were observed between AUC0-- t and BPC157 dosages, as well as between Cmax and BPC157 dosages (Figures 1D, E). The outright bioavailability after IM administration of each dosage was 18.82%, 14.49%, and 19.35%, respectively. After repeated IM management of BPC157 at 100 μg/ kg for 7 consecutive days, the plasma concentration versus time contour (Figure 1C) and pharmacokinetic criteria (Table 3) were similar to those observed after a single IM injection at a dose of 100 μg/ kg, with the exception of a slight rise in Cmax and AUC0-- t. The abovementioned results showed that BPC157 reached its optimal swiftly in rats and was quickly gotten rid of after reaching its height. A camera attached to a VMS-004 Discovery Deluxe USB microscopic lense (Veho, USA) was made use of for recording. In deeply anesthetized rats, laparatomized prior to sacrifice, we analyzed the gross sores in the intestinal system and in the belly (amount of the longest sizes, mm) (Gojkovic et al., 2020; Kolovrat et al., 2020; Gojkovic et al., 2021a; Knezevic et al., 2021a; Knezevic et al., 2021a; Gojkovic et al., 2021b; Knezevic The original source et al., 2021b; Strbe et al., 2021). The ordinary recovery prices of total radioactivity in pee, feces, and cage cleansing liquid gathered from 0 to 72 h after [3H] BPC157 administration in intact rats were 15.88% ± 2.99%, 2.25% ± 0.67%, and 1.41% ± 1.04%, respectively, and the proportion of recurring radioactivity in the cadavers was 54.31% ± 3.04% (Table 7; Number 3B). In addition to venous occlusion-induced lesions (Vukojevic et al., 2018; Gojkovic et al., 2020; Kolovrat et al., 2020), BPC 157 is understood to reduce lesions in the whole stomach system (Sikiric et al., 1994; Ilic et al., 2009; Cut et al., 2009; Ilic et al., 2010; Ilic et al., 2011a; Ilic et al., 2011b; Petrovic et al., 2011; Lojo et al., 2016; Drmic et al., 2017; Becejac et al., 2018). Furthermore, BPC 157 might reduce lesions in the liver (Sikiric et al., 1993b; Ilic et al., 2009; Ilic et al., 2010; Ilic et al., 2011a; Ilic et al., 2011b; Lojo et al., 2016; Drmic et al., 2017), consisting of liver cirrhosis, caused by bile duct ligation (Sever et al., 2019) or constant alcohol intake (Prkacin et al., 2001). Likewise, BPC 157 might protect against and turn around persistent cardiac arrest generated by doxorubicin application (Lovric-Bencic et al., 2004). BPC 157 decreases different arrhythmias (i.e., potassium overdose-induced hyperkalemia (Barisic et al., 2013), digitalis (Balenovic et al., 2009), neuroleptics (i.e., prolonged QTc-intervals that may likewise be centrally relevant) (Strinic et al., 2017), bupivacaine (Zivanovic-Posilovic et al., 2016), lidocaine (Lozic et al., 2020), and succinylcholine (Stambolija et al., 2016)). As a lately reviewed topic (Vukojevic et al., 2022), BPC 157 has been shown to minimize mind sores, trauma-induced brain injury (Tudor et al., 2010), compression-induced spine injury (Perovic et al., 2019), and stroke (Vukojevic et al., 2020). On top of that, BPC 157 lowers serious encephalopathies (NSAID overdose, Ilic et al., 2010; Ilic et al., 2011a; Ilic et al., 2011b; Lojo et al., 2016; Drmic et al., 2017), neurotoxin cuprizone-induced numerous sclerosis in a rat design (Klicek et al., 2013), and magnesium overdose (Medvidovic-Grubisic et al., 2017)). Alternatively, utilizing esketamine anesthesia (40 mg/kg esketamine (Rotexmedica, Germany) and 10 mg/kg diazepam (Apaurin; Krka, Slovenia) intraperitoneally), we caused abdominal area syndrome as explained before and kept high stomach pressure at 25 mmHg for 120 minutes prior to sacrifice. Medication (BPC 157 (10 µg or 10 ng/kg sc) or saline (5 ml)) was given after 10 min of high abdominal stress. Hence, we evaluated BPC 157 therapy as an alleviative concept in rats with well-known irreversible intra-abdominal hypertension. As verification, we used the crisis that accompanied the high intra-abdominal pressure-induced disorder, in which intra-abdominal high blood pressure all at once affected all abdominal vessels and body organs for a substantial duration and limited the ability to hire alternative paths, such that a dangerous circumstance was produced prior to therapy initiation. Evaluations were carried out at 1, 4, 7, 15, 30, 90, 180, and 360 days after injury. The chemotactic mobility of HUVECs was identified making use of transwell migration chambers (Corning) with 6.5 mm diameter polycarbonate filters (8 μm pore size), as defined formerly.28 Briefly, the lower chambers were full of 750 mL of RPMI 1640 tool including all supplements. HUVECs (3 × 104 cells per well) were seeded in leading chambers with DMSO or numerous doses of BPC-157 (1 μg/ mL, 5 μg/ mL, and 10 μg/ mL) in 500 mL RPMI 1640 with 0.5% FBS. Nonmigrated cells were gotten rid of with cotton swabs, and moved cells were repaired with ice-cold methanol and tarnished with 4 ′,6- diamidino-2-phenylindole (DAPI).Rewinding the Clock - Harvard Medical School
Rewinding the Clock.
Posted: Thu, 22 Mar 2018 07:00:00 GMT [source]

What body organs does BPC 157 recover?
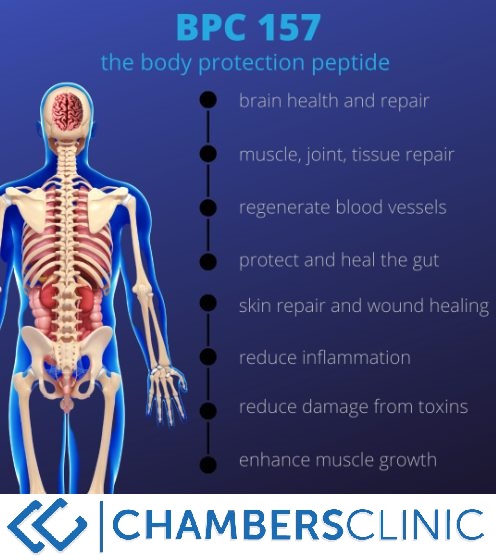
Research studies conducted in rodents and cultured cells have actually recommended that BPC-157 may support the recovery of numerous tissues, consisting of tendons, joints, nerves, the intestinal system, the belly, and skin. What are BPC-157''s primary disadvantages? BPC-157''s possible disadvantages are uncertain, provided the lack of human proof.
Social Links