
September 5, 2024
Can Tesofensine Deal With Obesity? Untangling The Secret Behind A Brand-new Weight Management Drug
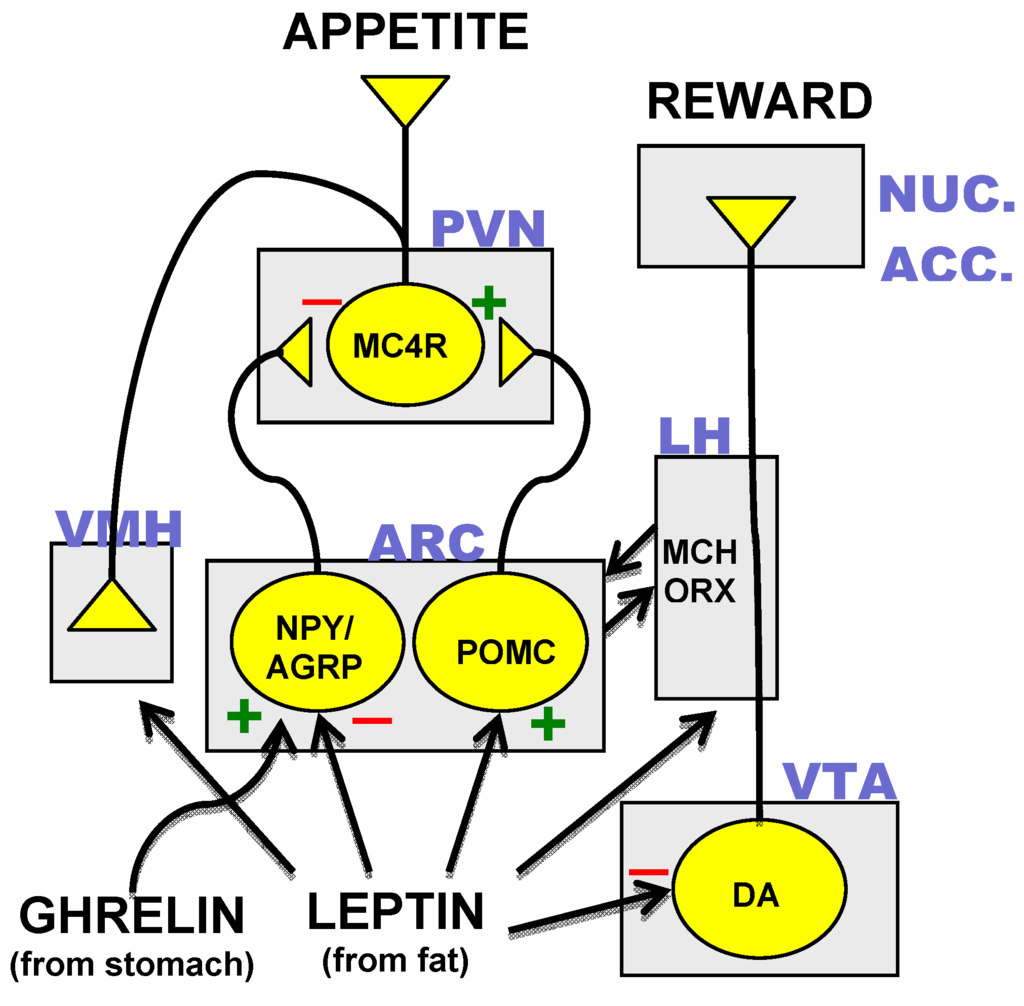
Tesofensine A Summary Frequency of weight problems in the US and Europe has actually reached epidemic degrees and, not surprisingly, has boosted the search for brand-new weight-loss medications. Glucagon-like peptide 1 receptor (GLP1R) agonism puts in both direct and indirect results on energy and glucose metabolic rate in vital peripheral organs as well as the mind. The worldwide excessive weight prevalence has actually almost tripled considering that 1975 and, within the United States, excess body weight affects more than 2 thirds of the population, with greater than one third of grownups and 20% of teenagers having obesity (see Related web links). A. It shows the efficiency of 4 rats in the sucrose discrimination task throughout sessions, expressed as a percent of right reactions. After five sessions, all subjects were able to distinguish between the different sucrose concentrations (above 75% proper for 3 consecutive days). Given that the half-life of tesofensine has to do with 8 days, we continued reviewing the rats' efficiency for 3 more days (S3 Fig, panel C).- A 2nd aim of this study, in computer mice, is to identify just how tesofensine targets LH GABAergic nerve cells to regulate feeding behavior.
- The glucagon household of receptors are activated by endogenous peptides making up growth hormone-releasing hormone, stomach repressive polypeptide (GIP), glucagon-like peptide 1 (GLP-1), glucagon-like peptide 2 (GLP-2), glucagon and secretin.
- Key Result Measures Coprimary end factors were the modifications from baseline in Unified Parkinson Illness Score Range (UPDRS) subscale II (activities of everyday living) plus subscale III (motor feature) complete score and in percentage of waking hours invested in "off" time kept in mind in self-scoring journals.
- A careful 5HT2C agonist, lorcaserin (ADP-356; Field), demonstrated efficiency in producing weight loss in stage II/III testing.
Melanocortin-4 Receptor Agonists
As part of the approval process, the FDA asked for that Orexigen, thesponsor, do a cardiovascular safety study to show that NB-32doesn' t increase major occasions as https://ewr1.vultrobjects.com/pharma-warehousing/Drug-recalls/product-strategy/detailed-clinical-weight-administration-university-of-utah.html figured out by a non-inferiority hazardratio of much less than 1.4. Orexigen enrolled 8,910 overweight and overweight topics inan end result research study, LIGHT, driven by the variety of significant cardio eventsincluding non-fatal stroke, non-fatal heart attack, and cardiovasculardeath. The test verified that after the 25% and 50% meantime evaluations ofevents, the non-inferiority hazard proportion was much less than 2.0. The sponsor brokethe blind and released confidential information midway via the test andinvalidated the outcomes prior to the noninferiority hazard ratio of 1.4 or lesswas gotten to, developing a need to duplicate the test under effectively blindedconditions [49]2 Anti-obesity Medications In Professional Advancement
However, whereas weight reduction effects usually convert from rats to people, optimum efficacy is traditionally 2 to four times reduced in humans relative to rodents (Fig. 3). It can be said that better family member weight-loss in rodents is expected as mice possess a greater mass-specific energy expense than people, with a greater payment of brownish fat to metabolic rate128. The high mass-specific metabolic price requires completely high caloric consumption to safeguard versus a persistent deficiency in energy equilibrium. It is consequently sensible that mice can consume food matching more than 10% of their body weight in a solitary day. Consequently, medicinal restraint of food consumption uses a bigger vibrant variety and more immediate impact on weight-loss in rodents about human beings.Which of the adhering to is an efficient therapy for obesity?

Social Links